Study of Go-Less® on female bladder voiding and patient’s satisfaction
• EFLA®940 Pumpkin seed extract
• SoyLife – Soy germ extract
Study summary
To investigate the efficacy and safety of Go-Less® on bladder control in women, a placebo-controlled, randomized clinical trial was conducted on women suffering from an overactive bladder in Korea.
Study design
Subjects: 120 women aged 35-70 years (60 placebo/60 test group).
Test substance: 500 mg Tablets containing 218.75 mg of Pumpkin seed extract (EFLA®940) and 31.25 mg of Soy germ extract (SoyLife).
Dosage: 2 tablets 2x per day
Duration: 12 weeks
Subjects were asked to maintain a healthy daily lifestyle including diet and exercise; health functional foods, medicines or non-medicine products that could affect the research results were prohibited.
A bladder diary was evaluated to measure the day and night amounts and frequency of urination and the frequency of urgency and incontinence. At each clinic visit flow rate and remaining urine were measured, a survey was conducted using OAB-q V8 (Overactive Bladder Questionnaire V8 Symptom Irritation scale) to evaluate the improvement of overactive bladder syndrome and quality of life, and SQoL-F (sexual quality of life questionnaire – female) to evaluate the improvement of the quality of sexual life. For safety evaluation, vital signs, blood and urine tests were performed at each visit. The presence of unexpected reactions and adverse effects were evaluated through interviews with the individual subjects.
Statistical analysis: Changes in blood, overactive bladder syndrome and urine dysfunction of each group by period were analyzed using a paired t-test, and a comparison between groups about the improvement level of each element at week 12 after taking the trial product was conducted through an unpaired t-test. In each statistical analysis, p<0.05 was considered to be statistically significant. Only those subjects that were compliant to the treatment and completed all measurements including the 12 weeks measurements were included in the statistical analyses for all time points.
Study of Go-Less® on female bladder voiding and patient’s satisfaction
Results
There is a significant decrease of urination frequency, urgency, incontinence and nocturia. These decreases are shown in the figures below.
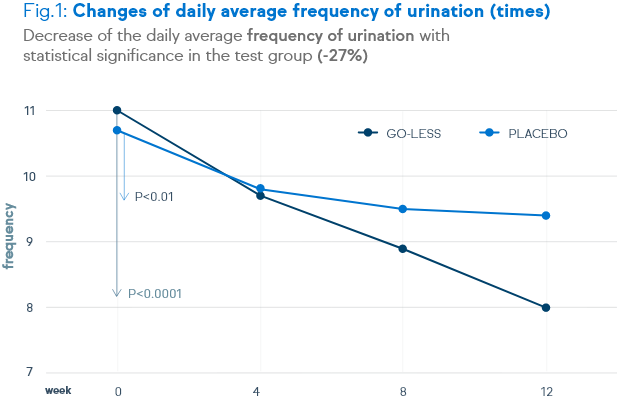
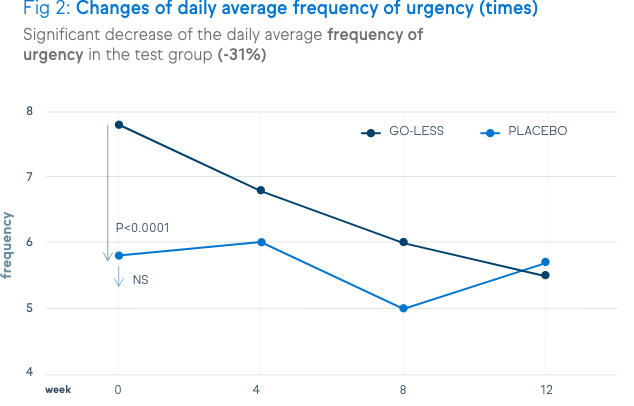
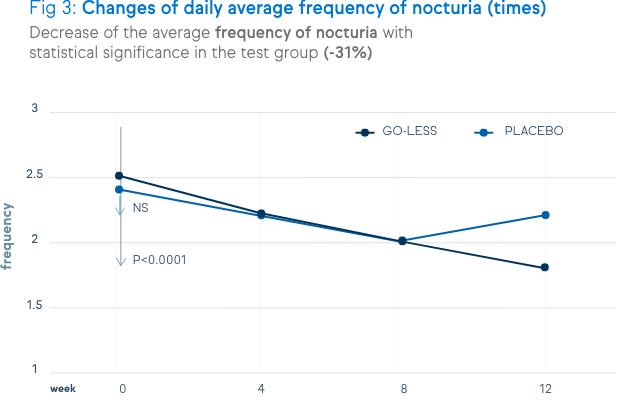
Not only symptoms of overactive bladder syndrome, but also the quality of life and the satisfaction level were positively assessed by the patients.
- significant improvement of quality of life (22%), measured on the
OAB-q V8 Symptom Irritation Index at week 12 - high satisfaction level of the treated patients: positive response
by 90.5% of the test group and will to continuation of therapy by 95.2%
(compared to 56.1% rsp. 46.3% of the placebo group)
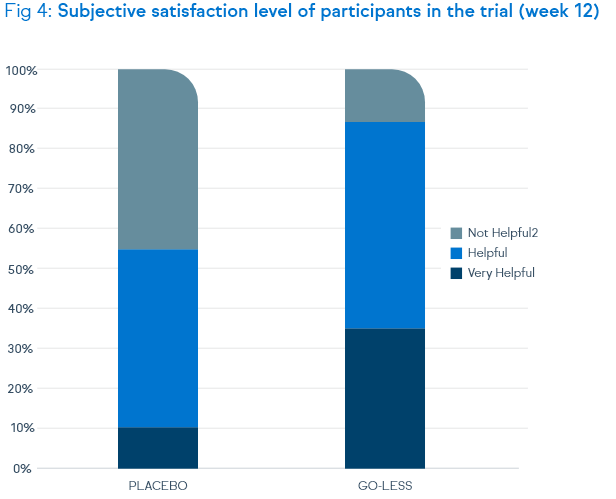
Go-Less® – High effectiveness in key symptoms of female overactive bladder syndrome and top level of patients’ satisfaction
Safety
– No identified safety concern with administration of Go-Less.
Conclusion
Since urgency has the biggest impact on quality of life in people with an overactive bladder, the improvement of the urgency score is a key element of success. In a clinical study, 12-week supplementation with Go-Less® significantly improved the average urgency score and frequency, compared to placebo.
Reference:
Shim et al. (2014) A randomized double-blind placebo-controlled clinical trial of a product containing pumpkin seed extract and soy germ extract to improve overactive bladder-related voiding dysfunction and quality of life. Journal of Functional Foods 8 (Supp C), 111-117.





